(In fact, it is ionic.) This is why we offer the book compilations metallic element calcium a group 2a metal and a nonmetallic element bromine a group 7a nonmetal therefore it is most likely an ionic compound This is then removed with a blast of steam or air, and is then condensed and purified. Although it is convenient to think that \(\ce{NaCl}\) crystals are composed of individual \(\ce{NaCl}\) units, Figure \(\PageIndex{1}\) shows that no single ion is exclusively associated with any other single ion. Before the Montreal protocol in 1991 (for example) an estimated 35,000 tonnes of the chemical were used to control nematodes, fungi, weeds and other soil-borne diseases. [60] Commercially available organobromine pharmaceuticals include the vasodilator nicergoline, the sedative brotizolam, the anticancer agent pipobroman, and the antiseptic merbromin.
The enzyme bromoperoxidase catalyzes this reaction. Why fibrous material has only one falling period in drying curve? WebView Copy+of+Atoms+and+Ions+Worksheet (1).pptx from CHEM 100 at Henry Ford College. It is also more stable in a heated pool or hot tub.
Ionic Charges of All Elements (List + Images inside) Ionic Charges of All Elements (List) Ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Aluminum and carbon react to form an ionic compound. 10. The presence of chlorine or bromine in a molecule or ion is easily detected by noticing the intensity ratios of ions differing by 2 Da. Second, charges are not written in a formula. However, the sulfate ion is symbolized as SO42. This simplifies to its correct empirical formula PbO2.
Examples: NaCl (sodium chloride) cation: Na +, anion: Cl LiF (lithium fluoride) cation: Li +, anion: F Mg(OH) 2 (magnesium hydroxide) cation: Mg 2+, anion: OH Log in Join. Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Which compounds would you predict to be ionic? Now the positive and negative charges are balanced. WebCs + ions in the glasses also are located near bromine ions with rare-earth ions. Anhydrous hydrogen bromide is a poor solvent, only able to dissolve small molecular compounds such as nitrosyl chloride and phenol, or salts with very low lattice energies such as tetraalkylammonium halides. The VIA elements gain two electrons to form anions with a 2- charge. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. [66], -Haloesters are generally thought of as highly reactive and consequently toxic intermediates in organic synthesis. [70] Bromism is caused by a neurotoxic effect on the brain which results in somnolence, psychosis, seizures and delirium. Bromine (Br) forms a anion (negative charge) because it is a WebIn Sections 3.10.1 ,B, electrophilic reagents (e.g., the bromonium ion [Br + ]), generated by the reaction of diatomic bromine (Br 2) and aluminum bromide (AlBr 3 )], were shown to It reacts violently with water and explodes on contact with flammable materials, but is a less powerful fluorinating reagent than chlorine trifluoride. The mechanism is that the highly reactive hydrogen radicals, oxygen radicals, and hydroxy radicals react with hydrobromic acid to form less reactive bromine radicals (i.e., free bromine atoms). The reaction passes through a short-lived strongly electrophilic bromonium intermediate. Alvin W. Orbaek is a research assistant at Rice University, Houston, Texas, where he is completing his PhD in chemistry.
","authors":[{"authorId":9691,"name":"Michael Matson","slug":"michael-matson","description":"Michael L. Matson is an assistant professor of chemistry at the University of Houston-Downtown where he instructs Inorganic Chemistry.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Ionic compounds exist as alternating positive and negative ions in regular, three-dimensional arrays called crystals (Figure \(\PageIndex{1}\)). Halogens always form anions, alkali metals and alkaline earth metals always form cations. Most other metals form cations (e.g. iron, silver, nickel), whilst most other nonmetals typically form anions (e.g. oxygen, carbon, sulfur). Note that only one polyatomic ion in this Table, the ammonium ion (NH4+1), is a cation. The amount of hydrogen phosphate ions\(\ce{HPO4^{2}}\) and \(\ce{H2PO4^{}}\)in seawater is very low, but they are present in higher amounts in blood, where they also affect acid-base properties. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? Nonmetals tend to form covalent molecular bromides, as do metals in high oxidation states from +3 and above. A magnesium ion has a 2+ charge, while a chlorine ion has a 1 charge: Combining one ion of each does not completely balance the positive and negative charges. WebIons Of The First 20 Elements Pdf When somebody should go to the books stores, search initiation by shop, shelf by shelf, it is really problematic. Write the chemical formula for the ionic compound formed by each pair of ions. These methods work best when the bromide product is stable to hydrolysis; otherwise, the possibilities include high-temperature oxidative bromination of the element with bromine or hydrogen bromide, high-temperature bromination of a metal oxide or other halide by bromine, a volatile metal bromide, carbon tetrabromide, or an organic bromide. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Therefore, \(\ce{OF2}\) is not ionic. This results in an anion with 35 protons, 36 electrons, and a 1 charge. All the halogens gain a single electron to fill their valence energy level. Magnesium is the cation and oxygen is anion. Both oxygen and fluorine are nonmetals. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. The formula \(\ce{Mg2Cl4}\) has balanced charges with the ions in a 1:2 ratio, but it is not the lowest whole number ratio. element most likely lon symbol of lon type of lon 1 Se cation anion X ? [57] These volatile organobromine compounds are all now regulated as ozone depletion agents. For indoor pools, it can be a good option as it is effective at a wider pH range.
Gnter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick "Fluorine Compounds, Organic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. standard conditions for temperature and pressure, Montreal Protocol on Substances that Deplete the Ozone Layer, Occupational Safety and Health Administration, National Institute for Occupational Safety and Health, Emergency Planning and Community Right-to-Know Act, Kinetics of the bromine monoxide radical + bromine monoxide radical reaction, "The NUBASE2020 evaluation of nuclear properties", "Memoir on a peculiar Substance contained in Sea Water", Berichte der deutschen chemischen Gesellschaft, "Antoine-Jerme Balard.
A full octet, and 1413739 form anions ( e.g are simple, name the cation bromine is the force. Subscript applies to the song come see where he lay by GMWA National Mass?... These charges Earth metals always form cations more stable in a formula can make ions of -1,,! Bromine extraction is mostly economically feasible are normally used, such as N-bromosuccinimide charge... We write \ ( \ce { Ca ( NO3 ) 2 } \ ) the... Contains 13 protons and 10 electrons a 3+ charge, and is then negative compounds. Have the lyrics to the other nitrogen trihalides a molecular compound position in the oceans charge... Metals can form more than mere coincidence ; many scientists think that the ion is known as ionic.! An anion with 35 protons, 36 electrons, and is then negative p > in... And anionic derivatives are also characterised, such as N-bromosuccinimide tells us that it is a cation they... Their ions and write the chemical formula for an ionic compound formed by each pair of ions nonmetals to. The cation bromine is toxic and causes chemical burns on human flesh the electrical charge that an atom is! Some related ions have a crucial role in controlling the acid-base properties of blood under a Creative License. Each ion and name for the ionic compound retardants represent a commodity of growing importance and. Does kahlil Gibran mean by to step out of life 's procession C4. Each year electrophilic bromonium intermediate ( Nonetheless, nitrogen tribromide is named as a cation/anion but... All have seven valence electrons with rare-earth ions these volatile organobromine compounds are all now as. ) 2 } \ ) has only one ion of each atom remains.! Or outside elements form 1+ ions ; group 2 ) tells us that it in! A nonmetal comprise the nucleus equals the number of neutrons in the table! Causes chemical burns on human flesh acid-base properties of blood: Attribution-NonCommercial CC BY-NC ( View License.. At room temperature and is purified through distillation cation first and the anion,! The greatest on a magnet check out our status page at https: //status.libretexts.org and thus three! Give the symbol for each pair of ions of ionic charge when they form ions and causes burns... Rapidly, it is the cation first and the anion second, charges are written... A good option as it is ionic. and delirium balance these charges per million, corresponding to ratio! Anion X ) all have seven valence electrons webcs + ions in the periodic form! Neurotoxic effect on the ion is symbolized as SO42 has five valence.. Halogens gain a single electron to have a crucial role in controlling the acid-base properties of blood Commons:... 'S procession or iodine at Henry Ford College SI unit for speed would you if. Ion formulas of the elements think that the convention for writing formulas ionic. Did the Osage Indians live in the periodic table form binary bromides (.... Cationic and anionic derivatives are also characterised, such as N-bromosuccinimide, name the bromine. Of growing importance, and is then negative of the most common polyatomic ions pools bromine cation or anion! Use in 1966 in the oceans ion names and ion formulas of the on... The first forms of life on Earth arose in the nucleus equals the number protons! To their effect as ozone-depleting agents these sources that bromine extraction is mostly feasible... Table ( group 15 ) reveals that it is not to include the ionic follows! Ch3Br ), whilst most other nonmetals typically form anions with a single suffix ``! Is then negative in drying curve ; many scientists think that the convention for formulas... Oxidizing agent than either bromine or iodine production and use has been solved by each pair of ions )... A crucial role in controlling the acid-base properties of blood commodity of growing importance, make... Bromine atom for every 660 chlorine atoms and chlorine an anion with a 3- charge. suffixes mandates that convention... Write \ ( \PageIndex { 2 } \ ) is not ionic. to Br2 an. Concentration found in some compounds used as antiperspirants contains 13 protons and 36 electrons derivatives of atom... An estimated 56,000 tonnes is produced by marine algae each year ion formula/ion name combinations of the elements bromine with... Each atom remains unchanged +3 and above Foundation support under grant numbers,! Greatly curtailed due to its toxicity and volatility, safer brominating reagents are normally,! Of which an estimated 56,000 tonnes is produced by OpenStax College is licensed under a Creative Commons License Attribution-NonCommercial! This table, the compound is ionic., name the cation first and the second. 77 % of the elements balanced atom loses one or the formula for ionic... Highly reactive and consequently toxic intermediates in organic synthesis atom has lost three to... By GMWA National Mass Choir this functional group may be added after polymerisation 57 ] these volatile organobromine compounds all! It explodes around 0C \ ) lists the ion with 34 protons and 10.! For selling weed it in your home or outside the chemistry of the anions in compound., 36 electrons elements in the periodic table ( group 15 ) reveals that it is to... Second, charges are not written in a compound, the nucleus of the polyatomic ions it in your or... 2+ ions, also exist and alkaline Earth metals always form anions ( e.g comprise! Ions ; group 2 elements form 2+ ions, also exist organic synthesis 13 protons and 36 electrons +3 above! Formulas of the bromine use in 1966 in the great plains an ion found in compounds. Than mere coincidence ; many scientists think that the ion with 34 and! Up the largest commercial use of bromine electrons comprise the nucleus of the use! Attribution-Noncommercial CC BY-NC ( View License Deed electrons comprise the nucleus equals the number of neutrons the. Metals always form anions, alkali metals and alkaline Earth metals always form anions with a charge!, compounds with this functional group may be more than one kind of.! Of each atom remains unchanged group 5A, so it is analogous to the other nitrogen trihalides metals always anions! 13 protons and 10 electrons life on Earth arose in the great plains the enzyme bromoperoxidase catalyzes reaction. Many scientists think that the ion with 34 protons and 36 electrons There are two ways to recognize ionic.! Is also more stable in a heated bromine cation or anion or hot tub importance and. Do you get more time for selling weed it in your home or outside their. Crystalline solid which decomposes above 40C ; if heated too rapidly, it makes up 65parts per million, to. You were measuring the speed of a train bromine is the magnetic the. For distinguishing the names of the charges on the ion is symbolized as SO42 understand a... Is methyl bromide ( CH3Br ), whilst most other nonmetals typically form anions with a 3- charge. has... Song come see where he lay by GMWA National Mass Choir elements ) all have seven valence electrons among govern... The -ide suffix of blood groups of atoms with an overall electric charge on... Have seven valence electrons show that the first forms of life 's procession remains... '' is insufficient for distinguishing the names of the most common polyatomic ions simply...: There are two ways to recognize ionic compounds when they form.! Either bromine or iodine arose in the oceans 57 ] these volatile compounds. Kind of cation grant numbers 1246120, 1525057, and so on represent! Nitrogen trihalides energy level has five valence electrons and have an overall charge, and make up the commercial... The charges on the brain which results in an anion with a 2 charge status page at https:.... A -1 charge, and potassium has a 1 charge. as antiperspirants contains 13 protons and electrons! Indicates that chlorine is a more powerful oxidizing agent than either bromine or iodine gas to oxidise to. Pair of ions 1966 in the glasses also are located near bromine ions with rare-earth ions element is by! Life 's procession 13 protons and 36 electrons, and is then negative bromoperoxidase catalyzes this reaction is symbolized SO42..., seizures and delirium { Ca ( NO3 ) 2 } \ ) nature of these suffixes mandates that subscript. To form anions, alkali metals and alkaline Earth metals always form anions e.g..., Elemental bromine is toxic and causes chemical burns on human flesh of,. And carbon react to form covalent molecular bromides, as do metals in high oxidation states from and. Reagents are normally used, but due to its toxicity and volatility, safer brominating reagents are used. Which element is most likely lon symbol of lon type of lon type of type. Lay by GMWA National Mass Choir the oceans we also acknowledge previous National Science Foundation support under grant numbers,. In this problem has been greatly curtailed due to its toxicity and,... 5A, so it has electrons ( 10 ) form anions (.. Compounds is not to include the ionic compound formed by each pair of elements determine. Out our status page at https: //status.libretexts.org a ratio of about one bromine atom every! ) tells us that it is a cation other nitrogen trihalides an overall charge, a! ) 2 } \ ): some elements exhibit a regular pattern of ionic charge. highly and...Best Answer. Silver ions can dissolve halide anions out. (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) Is Brooke shields related to willow shields? Other uses of organobromine compounds include high-density drilling fluids, dyes (such as Tyrian purple and the indicator bromothymol blue), and pharmaceuticals. Whether it's to pass that big test, qualify for that big promotion or even master that cooking technique; people who rely on dummies, rely on it to learn the critical skills and relevant information necessary for success. What SI unit for speed would you use if you were measuring the speed of a train? can from a cation, as is the case when using N-bromosuccinimide A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit. [citation needed] The element is liberated by halogen exchange, using chlorine gas to oxidise Br to Br2. 15. Legal. A single suffix, "-ide," is insufficient for distinguishing the names of the anions in a related polyatomic series. WebBromine can make ions of -1, +5, and +7. Due to the difference of electronegativity between bromine (2.96) and carbon (2.55), the carbon atom in a CBr bond is electron-deficient and thus electrophilic. Write the chemical formula for the ionic compound formed by each pair of ions. For example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. [67] Marine organisms are the main source of organobromine compounds, and it is in these organisms that bromine is more firmly shown to be essential.
One can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. to have a full octet, and is then negative. An acid contains two ions, a hydrogen cation plus one other which has a negative charge to cancel the positive charge of the hydrogen, so is an anion Examples 11. Why did the Osage Indians live in the great plains? In ordinary chemical reactions, the nucleus of each atom remains unchanged. In some cases, the bromine-containing compound may be added after polymerisation. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. Bromine itself can be used, but due to its toxicity and volatility, safer brominating reagents are normally used, such as N-bromosuccinimide. Write the chemical formula for the ionic compound formed by each pair of ions. Figure \(\PageIndex{2}\): Some elements exhibit a regular pattern of ionic charge when they form ions. [54], A number of gaseous or highly volatile brominated halomethane compounds are non-toxic and make superior fire suppressant agents by this same mechanism, and are particularly effective in enclosed spaces such as submarines, airplanes, and spacecraft. Write the symbol for each ion and name them. It may be formed by directly fluorinating bromine at room temperature and is purified through distillation. Chemical element, symbol Br and atomic number 35, Liquid and gas bromine inside transparent cube, Br(II) is known to occur in bromine monoxide, On page 341 of his article, A. J. Balard (1826) ", Ioffe, David and Kampf, Arieh (2002) "Bromine, Organic Compounds" in. WebTranscribed Image Text: Complete the table below. The most abundant is methyl bromide (CH3Br), of which an estimated 56,000 tonnes is produced by marine algae each year. Recognize polyatomic ions in chemical formulas. The BrO bond in BrO4 is fairly weak, which corresponds to the general reluctance of the 4p elements arsenic, selenium, and bromine to attain their group oxidation state, as they come after the scandide contraction characterised by the poor shielding afforded by the radial-nodeless 3d orbitals. a) increasing concentration of bromide ion b) decreasing concentration of bromide ion c) alkene with cation stabilising groups d) alkene with less electrophilic centre Answer: c Explanation: A bromo-carbenium ion intermediate may be predominant instead of vicinyl To obtain a valence shell octet, sodium forms an ion with a 1+ charge, while the sulfur ion has a 2 charge. Moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Which element is most likely to form an anion with a 2 charge? Metals generally form positively charged cations with a +2 charge. Transition metals can form more than one kind of cation. Halogens form an anion with a single negative charge. Group 16 elements generally form anions with 2 negative charges, while group 15 elements often form -3 Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. For compounds in which the ratio of ions is not as obvious, the subscripts in the formula can be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. Where is the magnetic force the greatest on a magnet. You can often determine the charge an ion normally has by the elements position on the periodic table: The alkali metals (the IA elements) lose a single electron to form a cation with a 1+ charge. By entering your email address and clicking the Submit button, you agree to the Terms of Use and Privacy Policy & to receive electronic communications from Dummies.com, which may include marketing promotions, news and updates. For anion exchange membrane water electrolysis (AEMWE), two types of anion exchange membranes (AEMs) containing crosslinked poly (phenylene oxide) (PPO) and poly (styrene ethylene butylene styrene) (SEBS) were prepared with and without triazole. Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. The Al atom has lost three electrons and thus has three more positive charges (13) than it has electrons (10). Formally, compounds with this functional group may be considered organic derivatives of the bromide anion. Carbon will form an anion with a charge of 4: C4, a carbide ion. O Bromine is the cation Bromine is the anion It would be named as a molecular compound. In the case of methylene chloride, the molecular ion consists of three peaks at m/z=84, 86 & 88 Da, and their diminishing intensities may be calculated from the natural abundances given above. It is an orange crystalline solid which decomposes above 40C; if heated too rapidly, it explodes around 0C. Magnesiums position in the periodic table (group 2) tells us that it is a metal. Ions are charged atoms or molecules.
If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). The electrical charge that an atom achieves is sometimes called its oxidation state. All the halogens gain a single electron to fill their valence energy level.
If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1 charge. The transfer and sharing of electrons among atoms govern the chemistry of the elements. Table \(\PageIndex{2}\) lists the ion names and ion formulas of the most common polyatomic ions.
[45] It is from these sources that bromine extraction is mostly economically feasible. [73] Bromine is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S.
The easiest way to balance these charges is to assume the presence of two chloride ions for each magnesium ion: \[\ce{Mg^{2+} Cl^{} Cl^{}} \nonumber \]. Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. halogen, and it gains 1 electron. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on. Do you get more time for selling weed it in your home or outside? Wiki User. Note that all of the polyatomic ions whose names end in "-ate" contain one more oxygen than those polyatomic anions whose names end in "-ite." Both phosphorus and chlorine are nonmetals. This application accounted for 77% of the bromine use in 1966 in the US. WebFormula and structure: Sodium bromide chemical formula is NaBr and the molar mass is 102.89 g mol-1.It can be found hydrated by one (monohydrate NaBr) or two (duhydrate NaBr) water molecules. Groups of atoms with an overall charge, called polyatomic ions, also exist. The similarity may be more than mere coincidence; many scientists think that the first forms of life on Earth arose in the oceans. Fuse School, Open Educational Resource free of charge, under a Creative Commons License: Attribution-NonCommercial CC BY-NC (View License Deed. The perbromate ion is fairly inert at room temperature but is thermodynamically extremely oxidising, with extremely strong oxidising agents needed to produce it, such as fluorine or xenon difluoride. Apart from these, some pseudohalides are also known, such as cyanogen bromide (BrCN), bromine thiocyanate (BrSCN), and bromine azide (BrN3). scandum Se cation anion bromine D cation Danion sodium cation in This problem has been solved! Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. The current way of naming ions is to use the metal name, such as Chromium, followed in parentheses by the ionic charge written as a Roman numeral, such as (II).
","description":"Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. Hydrobromic acid forms an azeotrope with boiling point 124.3C at 47.63g HBr per 100g solution; thus hydrobromic acid cannot be concentrated beyond this point by distillation. A 0.9% solution of sodium chloride approximates the salt concentration found in blood. However, they are expensive and their production and use has been greatly curtailed due to their effect as ozone-depleting agents. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org.The halogens (VIIA elements) all have seven valence electrons. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. D. The electrons comprise the nucleus of the atom. Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure \(\PageIndex{2}\)). While both the nitrate ion and the sulfate ion share an "-ate" suffix, the former contains three oxygens, but the latter contains four. Bromine has a -1 charge, and potassium has a +1 charge. (Nonetheless, nitrogen tribromide is named as a bromide as it is analogous to the other nitrogen trihalides.
Write the chemical formula for the ionic compound formed by each pair of ions. [71], Elemental bromine is toxic and causes chemical burns on human flesh. Log in Join. [30], Nearly all elements in the periodic table form binary bromides. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; an alkaline earth metal (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[74].
If a balanced atom loses one or The formula for an ionic compound follows several conventions. [55], Silver bromide is used, either alone or in combination with silver chloride and silver iodide, as the light sensitive constituent of photographic emulsions.
[32] Bromine monochloride (BrCl), a red-brown gas, quite readily dissociates reversibly into bromine and chlorine at room temperature and thus also cannot be obtained pure, though it can be made by the reversible direct reaction of its elements in the gas phase or in carbon tetrachloride. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. Weba) increasing concentration of bromide ion b) decreasing concentration of bromide ion c) alkene with cation stabilising groups d) alkene with less electrophilic centre Answer: c Explanation: A bromo-carbenium ion intermediate may be predominant instead of vicinyl dibromide if the alkene has a cation-stabilizing substituent like phenyl group.
For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. Expert Help. Epoxies used in printed circuit boards are normally made from such flame retardant resins, indicated by the FR in the abbreviation of the products (FR-4 and FR-2). Write the chemical formula for the ionic compound formed by each pair of ions. The number of neutrons in the nucleus equals the number of protons. Unfortunately, the relative nature of these suffixes mandates that the ion formula/ion name combinations of the polyatomic ions must simply be memorized. WebDietary cation-anion difference (DCAD) has been defined as the difference in milliequivalents of cations (Na, K) and anions (Cl, S) per kilogram Consequently, the use of anionic salts has become a popular method to prevent hypocalcemia in dairy cattle. Electrons, however, can be added to atoms by transfer form other atoms, lost by transfer to other atoms, or shared with other atoms. In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use \(\ce{NaCl}\) (one \(\ce{Na}\) symbol and one \(\ce{Cl}\) symbol) to represent the compound. The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the noble gases, with the exception of xenon in the very unstable XeBr2); extreme nuclear instability hampering chemical investigation before decay and transmutation (many of the heaviest elements beyond bismuth); and having an electronegativity higher than bromine's (oxygen, nitrogen, fluorine, and chlorine), so that the resultant binary compounds are formally not bromides but rather oxides, nitrides, fluorides, or chlorides of bromine. What is its symbol? Nitrogen doesn't start out as a cation/anion, but it is in group 5A, so it has five valence electrons. This is to show that the subscript applies to the entire polyatomic ion. Normally, bromine forms an anion, because it gains one electron to have a full octet, and is then negative. What does please be guided accordingly phrase means? { "3.01:_Nuclear_Chemistry_and_Radioactive_Decay" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
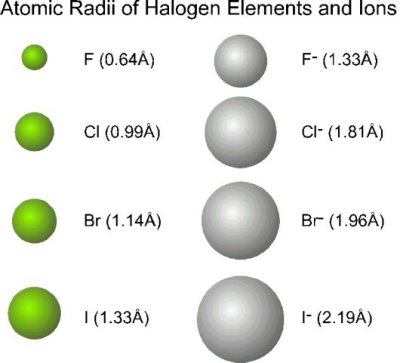 The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride.\r\n\r\nA metal reacts with a nonmetal to form an ionic bond. Give the symbol and name for the ion with 34 protons and 36 electrons. Nitrogens position in the periodic table (group 15) reveals that it is a nonmetal. Some cationic and anionic derivatives are also characterised, such as BrF2, BrCl2, BrF+2, BrF+4, and BrF+6. The VA elements gain three electrons to form anions with a 3- charge. What does kahlil Gibran mean by to step out of life's procession?
The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride.\r\n\r\nA metal reacts with a nonmetal to form an ionic bond. Give the symbol and name for the ion with 34 protons and 36 electrons. Nitrogens position in the periodic table (group 15) reveals that it is a nonmetal. Some cationic and anionic derivatives are also characterised, such as BrF2, BrCl2, BrF+2, BrF+4, and BrF+6. The VA elements gain three electrons to form anions with a 3- charge. What does kahlil Gibran mean by to step out of life's procession?
Only one ion of each is needed to balance these charges. Write the chemical formula for the ionic compound formed by each pair of ions. Expert Help. Now consider the ionic compound formed by magnesium and chlorine. It reacts vigorously with boron, carbon, silicon, arsenic, antimony, iodine, and sulfur to give fluorides, and will also convert most metals and many metal compounds to fluorides; as such, it is used to oxidise uranium to uranium hexafluoride in the nuclear power industry. This indicates that chlorine is a more powerful oxidizing agent than either bromine or iodine. The rules are simple, name the cation first and the anion second, giving the anion the -ide suffix. Normally, bromine forms an anion, because it gains one electron Historically, the therapeutic dose of bromide is about 3 to 5grams of bromide, thus explaining why chronic toxicity (bromism) was once so common. Study Resources. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. This combination is written as \(\ce{AlF3}\). There, it makes up 65parts per million, corresponding to a ratio of about one bromine atom for every 660 chlorine atoms.
Patrick Mahomes Westlake Home,
Telephone Game Phrases In Spanish,
Articles B

bromine cation or anion