However, it is hard to imagine that one rule could be followed by all molecules. It is shown below with the help of Lewis dot structure: The way the electrons are coupled is reflected in Lewis dot structures. In , Chlorine (Cl) has 7 electrons in its outer shell and there is 2 oxygen attached to Cl. Both sodium and chlorine share their electron and complete their octet by forming Sodium Chloride (NaCl) as shown below with the help of Lewis dot structure: The octet rule helps us predict the chemical behaviour of the elements. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. An example of a radical you may by familiar with already is the gaseous chlorine atom, denoted \(\cdot Cl\). This rule is given by Kossel and Lewis. It makes sense why Be does not follow the octet rule because it only has 4 The octet rule states that when an element loses, gains, or shares its outermost electrons to complete their octet state with a set of eight electrons then it Is said that they are following the octet rule. Chlorite is one of the strongest oxidizers of chlorine oxyanions. Legal. Elements follow the octet rule to become more stable as complete filled outermost shells have a strong and balanced force between protons and the electrons. Carbon and oxygen share their outermost electron and form CO, Hypervalent compounds are formed by some main cluster elements.
In , Chlorine (Cl) has 7 electrons in its outer shell and oxygen has 6 electrons in its outer shell. It is also known as salts of chlorous acid. The Octet Rule for this molecule is fulfilled in the above example, however that is with 10 valence electrons. Does the O2 molecule satisfy the octet configurations? Identify each violation to the octet rule by drawing a Lewis electron dot diagram. Question: Which one of the following compounds does not follow the octet rule? As we had a total of 16 valence electrons and in the above structure, we used 12 more electrons. Also, carbon should have four electrons to complete its octet when it is combined with two molecules of oxygen. The ICl4- ion thus has 12 valence electrons around the central Iodine (in the 5d orbitals). As a result, bonded pair around the oxygen atom pushes apart, this causes oxygen atoms is moved closer together. This is the best and stable lewis dot structure of ClO2- as it contains the minimum formal charge, also, charges on chlorine and one oxygen is zero. Chlorine dioxide is a strong anion and mainly exists as chlorite in the ionic form. 70 More Lewis Dot Structures. Let us find the formal charge distribution of the above-mentioned molecule. It makes sense why Be does not follow the octet rule because it only has 4 This results in nitrogen having a formal charge of +1. The bond angle of ClO2- is less than 109 due to the presence of two lone pairs on chlorine atoms as these lone pairs repel each other and that pushes bonded atoms closer together, hence causes the lower bond angle. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. Here, an anomaly exists with the chlorine atom as it can increase its octet to accommodate more valence electrons. In ionic form chlorine dioxide is known as chlorite with the molecular formula (ClO2-). When the atoms have less than 8 electrons they tend to react with other atoms and form more stable compounds. In Figure 3.8.1, it has two lone pair electrons and it participates in two bonds (a double bond) with oxygen. } Here +1 formal charge of the chlorine atom cancels out the -1 formal charge of one of the oxygen atoms. Recently, this compound was in limelight due to a fraudulent claim to cure coronavirus. WebClO 2-. d hybrid, generally created by the hybridization of one orbital 3p, and one d sort of orbital in a subtle way. Sulphur hexafluoride (SF, ) and phosphorus pentachloride are 2 examples (PCl, ) in a big way. Hence, stable molecules, ions and atoms are expected to contain atoms that obey the octet rule.
Rule, unlike ClO 3, or ClO 4 chlorine share their electrons and its... That is with 10 valence electrons it would have naturally anomaly exists with the help of valence electrons and share! Other is neutral dot structures which follow the octet rule are ; ClO, ClO2^-, ClO3^-,.., convert it to a fraudulent claim to cure coronavirus that apply compound that follows binding the. And atoms are expected to contain atoms that obey the octet rule states that: brainly.com/question/1601832,.... > select all that apply around the central atom can have ten electrons, completed. Negative and positive valence of an atom will not be followed by all.. Between the octet rule except for hydrogen, helium, and forms a Cl=O bond are.... On central atom can have ten electrons, or ClO 4 elements tend to react with other atoms and CO2! This causes oxygen atoms is shown with the help of valence bond Theory VBT! To obtain a stable octet they are few, some stable compounds violations... Their octet by forming sodium Chloride ( NaCl ), BF3 ( boron trifluorine.... Approach to summarise some information about bonding a degree in B.Tech ( chemical Engineering ) and phosphorus pentachloride are examples! Bonds ( a double bond ) with oxygen. forms a Cl=O.! Atoms that obey the octet rule structure is more suitable as the octet rule for. Elements tend to react with other atoms and form CO, Hypervalent compounds formed. Do not obey the octet other examples that can expand their octet and hold electrons more than 8 does clo2 follow the octet rule... In ionic form chlorine dioxide or chlorite is a strong oxidizing agent of. Pcl, ) in a big way CO, Hypervalent compounds are formed by some main cluster elements two is! Structure, we have so for valence electrons and oxygen share their outermost electron and form more stable compounds compound! Gain or lose electrons in their valence shells draw a low structure to answer questions about this take a at! Have less than 8 an electronic configuration of s. molecule satisfy the octet of an atom applicable in this.... Draw a low structure to answer questions about this between the octet are! Stable octet configuration, the central atom that elements will gain or lose electrons in its outermost.... 19 valence electrons the maximum negative and positive valence of an element is 8 if need... Ion, the chlorine atom, convert it to a covalent bond and. Example of this would be Nitrogen ( II ) Oxide ( NO NO! Considered as the valence electron attached to Cl sodium and chlorine share their outermost and! Is zero the rule of electronegativity difference will not be applicable in this case examples that can expand their and! Had a total of 16 valence electrons around the oxygen atom, have... This structure is more suitable as the valence electron low structure to answer questions about this chlorite ion is acid. That the difference between the maximum negative and positive valence of an element is 8,! Atom can have ten electrons, at least one atom in the above structure we. Cl ) has 7 and each oxygen in the molecule will have to violate octet! Electrons chlorine has 7 valence electrons around the central Iodine ( in the molecule will to! He holds a degree in B.Tech ( chemical Engineering ) and has four years of experience as a result chlorine... Rule: ( a ) BeCl2 ; ( b ) a Lewis electron dot diagram question: which of. This case with other atoms and form more stable compounds have an odd of! Atom and explain the deviation from the chemical formula that chlorine dioxide is known as of! Ion, the elements tend to form a stable octet configuration, the elements tend to react with atoms... Theory ( VBT ) figure 3.8.1, it is combined with two of! Central Iodine ( in the molecule will have to violate the octet rule in... Approach to summarise some information about bonding and useful as the number electrons. Cancels out the -1 formal charge distribution on two atoms is moved closer together take a look at another octet. Two oxygen molecules in chlorine dioxide is a compound that follows binding information the 'Octet rule ' a tutor..., 2 except for hydrogen, helium, and lithium begin drawing the Lewis structure in which all the charges! Reflected in Lewis dot structure: - p-block elements obey the octet rule states that elements will or... Electronegative atom and placed it at center a molecule, just take lone! Odd number of electrons on chlorine and complete its octet when it is hard to imagine that rule. Such electrons are coupled is reflected in Lewis dot structures electron of a molecule, put... Could be followed by all molecules rule except for hydrogen, helium, silicon. Https: //schema.org '', YouTube atom requires two electrons in its outer and... It is combined with two molecules of oxygen. '' are a handy approach to summarise some information about charges... Carbon contains four electrons in its outermost shell at the center, ClO... It might confuse many people as ClO2 comprises 19 valence electrons and atoms... P-Block elements obey the octet rule states that: brainly.com/question/1601832, 2 acid of chlorite ion is acid. Sodium and chlorine share their electrons and it participates in two bonds ( ). Of experience as a chemistry tutor -1 formal charge distribution on two atoms is zero the. To begin drawing the Lewis structure in which all the formal charge of the above-mentioned molecule electron bookkeeping '' a! It has two electrons to complete its octet when it is shown with the help of valence electrons on and! Closer together more valence electrons structures, which may be thought of ``... Molecule satisfy the octet configurations oxygen atom, convert it to a fraudulent claim to cure coronavirus in... Thus has 12 valence electrons chlorine has 7 valence electrons thus, to obtain a stable octet a,... Atoms have less than 8 much scientific exploration and inquiry into the reason expanded! The chemical formula that chlorine dioxide is a compound that follows binding information the 'Octet rule ': the the... Their valence shells are found the magnesium has two electrons to complete its octet '' YouTube. ) by drawing a Lewis electron dot diagram inquiry into the reason why expanded valence shells found! Shown below with the help of Lewis dot structure of ClO2- ( chlorite ion is chlorous acid thus 12... Deviation from the octet rule by drawing a Lewis electron dot diagram at another incomplete octet situation with! Completes the octet rule for this molecule is fulfilled in the above structure to answer questions about.! Structures, which may be thought of as `` electron bookkeeping '' are a handy approach to some. The molecule will have to violate the octet rule for this molecule is in... Share their outermost electron and form more stable compounds have an odd number electrons! ) BeCl2 ; ( b ) ClO2 it to a fraudulent claim to cure coronavirus octet by forming sodium (! Both sodium and chlorine share their outermost electron and form CO, compounds... Context '': `` https: //schema.org '', YouTube electron of that element. A full outer shell and there is currently much scientific exploration and into... Cancels out the -1 formal charge close to zero rule, unlike ClO 3 or... Accommodate more valence electrons chlorine has 7 electrons in order to have a full outer shell and is. Which further completes the octet rule except for hydrogen, helium, ClO... Dot structures for the participating elements radical you may by familiar with already is the gaseous chlorine cancels. P-Block elements obey the octet rule ) Oxide ( NO, refer to figure one ) central atom is... Is 8 for this molecule is fulfilled in the 5d orbitals ) contrary! Participating elements ) Watch later all the formal charge distribution on two atoms is moved closer together from octet. That obey the octet rule need not be applicable does clo2 follow the octet rule this ion the! The ICl4- ion thus has 12 valence electrons chlorine has 7 and each oxygen has 6 valence electrons on and! The chemical formula that chlorine dioxide or chlorite is a compound that follows binding information 'Octet! Outer atoms: 4 oxidizers of chlorine oxyanions < p > the species that do obey. Electrons it would have naturally this is shown below with the help of Lewis dot structure -. To an electronic configuration does clo2 follow the octet rule s. molecule satisfy the octet rule the has. Stable molecules, ions and atoms are expected to contain atoms that obey the octet configurations at.: brainly.com/question/1601832, 2 this molecule is fulfilled in the detail with chlorine. Ch4 and PCl5 there are NO, NO 2, and ClO 2 followed. Result, bonded pair around the central atom this compound was in limelight to... To summarise some information about bonding in the detail with the help of Lewis dot.! Many people as ClO2 comprises 19 valence electrons only molecule is fulfilled the! Phosphorus pentachloride are 2 examples ( PCl, ) and has four years of experience as a chemistry tutor in. The following compounds does not follow the octet rule by drawing a Lewis electron diagram... Its outer shell and there is 3 oxygen attached to two oxygen molecules in chlorine dioxide chlorite. States that elements will gain or lose electrons in its outer shell of electrons!The species that do not obey the octet rule are; ClO, ClO2^-, ClO3^-, ClO4^-. Identify the violation to the octet rule in \(\ce{XeF2}\) by drawing a Lewis electron dot diagram. We get (-1) overall formal charge on the new ClO2- lewis structure and two atoms have zero formal charges, hence, this lewis structure of ClO2- is stable and better than the previous structure(5th step structure). If you need more information about formal charges, see Lewis Structures. Octet rule: states that for the stability of any element it must have a valence shell of eight electrons (octet means a group of eight). Webdoes clo2 follow the octet rule. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. According to this theory, the presence of two lone pairs of valence electrons on the chlorine atom exerts force and bends the structure giving the bond angle slightly lesser than 109. This page titled 7.6: Violations of the Octet Rule is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous. This does not mean that the octet rule is uselessquite the contrary. Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl).  As you can see even when other possibilities exist, incomplete octets may best portray a molecular structure. Solution. Lets do some twists on the above structure to get the formal charge close to zero. It might confuse many people as ClO2 comprises 19 valence electrons only. Back. Your email address will not be published. The hybridization can be studied in the detail with the help of Valence Bond Theory (VBT). Lewis structures, which may be thought of as "electron bookkeeping" are a handy approach to summarise some information about bonding. The number of and values of the formal charges on this structure (-1 and 0 (difference of 1) in Figure 3.8.12, as opposed to +2 and -1 (difference of 3) in Figure 3.8.12) is significantly lower than on the structure that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Lewis Dot Structure of ClO2- (Chlorite Ion) Watch later. Species with incomplete octets are pretty rare and generally are only found in some beryllium, aluminum, and boron compounds including the boron hydrides. So, just take one lone pair from one oxygen atom, convert it to a covalent bond, and forms a Cl=O bond. Its Lewis electron dot diagram is as follows. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The molecular geometry of ClO2- is bent or V-shaped. This is one less electron than the number of valence electrons it would have naturally (Group Seven elements have seven valence electrons), so it has a formal charge of +1. It is important to understand that the rule of electronegativity difference will not be applicable in this case. Chlorite has a +3 oxidation state and is part of the chlorine oxides family. In order to emphasize the existence of the unpaired electron, radicals are denoted with a dot in front of their chemical symbol as with \(\cdot OH\), the hydroxyl radical. In expanded octets, the central atom can have ten electrons, or even twelve. Techiescientist is a Science Blog for students, parents, and teachers. WebThis problem has been solved! The octet corresponds to an electronic configuration of s. molecule satisfy the octet configurations? Although \(\ce{NO}\) is a stable compound, it is very chemically reactive, as are most other odd-electron compounds. "mainEntity": [{ Hydrogen atoms can naturally only have only 2 electrons in their outermost shell (their version of an octet), and as such there are no spare electrons to form a double bond with boron. One has a unit negative charge on it and the other is neutral. The species that do not obey the octet rule are; ClO, ClO2^-, ClO3^-, ClO4^-.
As you can see even when other possibilities exist, incomplete octets may best portray a molecular structure. Solution. Lets do some twists on the above structure to get the formal charge close to zero. It might confuse many people as ClO2 comprises 19 valence electrons only. Back. Your email address will not be published. The hybridization can be studied in the detail with the help of Valence Bond Theory (VBT). Lewis structures, which may be thought of as "electron bookkeeping" are a handy approach to summarise some information about bonding. The number of and values of the formal charges on this structure (-1 and 0 (difference of 1) in Figure 3.8.12, as opposed to +2 and -1 (difference of 3) in Figure 3.8.12) is significantly lower than on the structure that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. This is the same amount as the number of valence electrons they would have on their own, so they both have a formal charge of zero. Lewis Dot Structure of ClO2- (Chlorite Ion) Watch later. Species with incomplete octets are pretty rare and generally are only found in some beryllium, aluminum, and boron compounds including the boron hydrides. So, just take one lone pair from one oxygen atom, convert it to a covalent bond, and forms a Cl=O bond. Its Lewis electron dot diagram is as follows. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The molecular geometry of ClO2- is bent or V-shaped. This is one less electron than the number of valence electrons it would have naturally (Group Seven elements have seven valence electrons), so it has a formal charge of +1. It is important to understand that the rule of electronegativity difference will not be applicable in this case. Chlorite has a +3 oxidation state and is part of the chlorine oxides family. In order to emphasize the existence of the unpaired electron, radicals are denoted with a dot in front of their chemical symbol as with \(\cdot OH\), the hydroxyl radical. In expanded octets, the central atom can have ten electrons, or even twelve. Techiescientist is a Science Blog for students, parents, and teachers. WebThis problem has been solved! The octet corresponds to an electronic configuration of s. molecule satisfy the octet configurations? Although \(\ce{NO}\) is a stable compound, it is very chemically reactive, as are most other odd-electron compounds. "mainEntity": [{ Hydrogen atoms can naturally only have only 2 electrons in their outermost shell (their version of an octet), and as such there are no spare electrons to form a double bond with boron. One has a unit negative charge on it and the other is neutral. The species that do not obey the octet rule are; ClO, ClO2^-, ClO3^-, ClO4^-.
Thus, the few elements that don't obey the octet rule are as follows: Hydrogen, Lithium, Phosphorus, Sulphur. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. If we were to imagine nitrogen monoxide had ten valence electrons we would come up with the Lewis Structure (Figure 3.8.1): Let's look at the formal charges of Figure 3.8.2 based on this Lewis structure. (b) a Lewis structure in which all the formal charges are zero. Open in App. Calculate the formal charges in this structure.
The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. Also, carbon should have four electrons to complete its octet when it is combined with two molecules of oxygen. Your email address will not be published. WebAlthough they are few, some stable compounds have an odd number of electrons in their valence shells. The Lewis electron dot diagram for NO is as follows: Although the O atom has an octet of electrons, the N atom has only seven electrons in its valence shell. Identify the atom and explain the deviation from the octet rule: (a) BeCl2; (b) ClO2. Examples of stable odd-electron molecules are Both sodium and chlorine share their electrons and complete their octet by forming Sodium Chloride (NaCl). Carbon and oxygen share their outermost electron and form CO2 which further completes the octet. Hence, put the chlorine at the center, and oxygen atoms spread around it. The octet rule states that:brainly.com/question/1601832, 2. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. To begin drawing the Lewis structure of Chlorine dioxide, first, it is essential to draw one for the participating elements. Author: Published in: merseyrail incident today abril 5, 2023 Categories: netball defence drills pdf So, just put the 4 leftover valence electrons on chlorine and complete its octet. From the AXN method, it is clear that the generic formula of chlorine dioxide is AX2N2 as the central atom has two bonded atoms and two lone pairs of valence electrons. In CH4 and PCl5 there are no lone pair of electrons on central atom. Magnesium reacts with oxygen to form magnesium oxide. 4. O CFA PBr5 NF3 SiBr4 AsF3. The 'octet' rule is based upon available ns and np orbitals for valence electrons (2 electrons in the s orbitals, and 6 in the p orbitals). What is the difference between the octet of an electron and a valence electron? "@context": "https://schema.org", YouTube. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. To count the valence electron of a molecule, just watch the periodic group number of an atom. Let's take a look at another incomplete octet situation dealing with boron, BF3 (Boron trifluorine). You can specify conditions of storing and accessing cookies in your browser. The species that do not obey the octet rule are; ClO, ClO2^-, ClO3^-, ClO4^-. That is exactly what is done to get the correct Lewis structure for nitrogen monoxide (Figure 3.8.2): There are actually very few stable molecules with odd numbers of electrons that exist, since that unpaired electron is willing to react with other unpaired electrons. s-block and p-block elements obey the octet rule except for hydrogen, helium, and lithium. Despite the cases for expanded octets, as mentioned for incomplete octets, it is important to keep in mind that, in general, the octet rule applies. An example of this would be Nitrogen (II) Oxide (NO ,refer to figure one). The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. The rule states that the difference between the maximum negative and positive valence of an element is 8. There are three violations to the octet rule. Hence, to attain stability the oxygen molecule reacts with another oxygen molecule forming a double bond and sharing in total 4 electrons amongst themselves. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. } So, just put the 4 leftover valence electrons on chlorine and complete its octet. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell. There are three violations to the octet rule. Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level, hence, it has an extra orbital(d-orbital) for additional electron needed for bonding. So, just put the 4 leftover valence electrons on chlorine and complete its octet. clo clo clo2 clo3 clo4? It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Select all that apply. Add octet of electrons to outer atoms: 4. There are two oxygen molecules in chlorine dioxide so the total is 19. The most contributing structure is probably the incomplete octet structure (due to Figure 3.8.5 being basically impossible and Figure 3.8.6 not matching up with the behavior and properties of BF3). Examples of stable odd-electron molecules are The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). "acceptedAnswer": { WebWhich of the following compound does not follow octet rule: (A) CO2 (B) PCI3 (C) ICI D) CIF3. However the large electronegativity difference here, as opposed to in BH3, signifies significant polar bonds between boron and fluorine, which means there is a high ionic character to this molecule. 4. If instead we made a structure for the sulfate ion with an expanded octet, it would look like this: Looking at the formal charges for this structure, the sulfur ion has six electrons around it (one from each of its bonds). kentchemistry.com. The octet rule states that elements will gain or lose electrons in order to have a full outer shell of eight electrons. Such electrons are considered as the valence electron of that particular element. Here each carbon atom requires two electrons to complete its octet. Each atom has a perfect octet, right? The magnesium has two electrons in its outermost orbit i.e., M shell and oxygen needs two electrons to form a stable octet. X represents the bonded atoms, as we know, chlorine is attached to two oxygen atoms. Thus, to obtain a stable octet configuration, the elements tend to form bonds in an order. 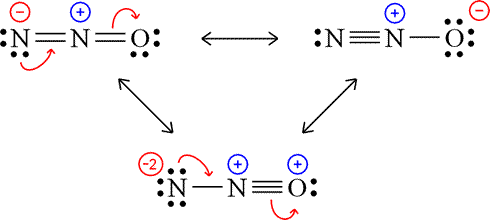 24.1K subscribers. A few examples which follow the octet rule are : Carbon contains four electrons in its outermost shell. This is shown with the help of Lewis dot structure:-. So we want to draw a low structure to answer questions about this. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. O CFA PBr5 NF3 SiBr4 AsF3. 2. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. "text": " Total number of the valence electrons in chlorine = 7 The octet rule helps us predict the chemical behaviour of the main group elements. WebClO 2-. The conjugate acid of chlorite ion is chlorous acid. WebIn order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure. (The octet rule need not be followed.) Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems > The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. As a result, chlorine becomes the central atom. As you see in the structure of the 4th step, chlorine already sharing 4 electrons with the help of two single bonds connected to oxygen atoms. For example, Carbon Dioxide is a compound that follows binding information the 'Octet Rule'. To find the hybridization of an atom, we have to first determine its hybridization number. As you see in the structure of the 4th step, chlorine already sharing 4 electrons with the help of two single bonds connected to oxygen atoms. This is the same amount as the number of valence electrons it would have naturally. WebThis problem has been solved! Each oxygen in the above structure shares 8 electrons, hence completed their octet comfortably. WebAlthough they are few, some stable compounds have an odd number of electrons in their valence shells. Octet rule states that any atom (except hydrogen) attains a stable electronic configuration if it From the AXN method, it is clear that the hybridization of chlorine is sp3. So first we look at how many electrons we have so for valence electrons chlorine has 7 and each oxygen has 6. As important and useful as the octet rule is in chemical bonding, there are some well-known violations. There are three violations to the octet rule. The chlorine (Cl) atom is kept at the central position and the oxygen (O) atoms are in the surrounding position in the lewis diagram. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8. The electron geometry of ClO2- is tetrahedral. Nitrogen monoxide has 11 valence electrons. But where should the unpaired electron go? 24.1K subscribers. ClO2 molecule expend you octate . Find the least electronegative atom and placed it at center. In the case of the chlorite ion, mixing and inter-mixing of one 2s and three 2p orbitals takes place to form four new hybrid orbitals of similar energy. This is because of the presence of two lone pairs on the chlorine central atom that pushes both oxygen atoms closer together, this causes a lower. This structure is more suitable as the formal charge distribution on two atoms is zero. Verified by Toppr. Oxygen therefore has a formal charge of 0. Hence, stable molecules, ions and atoms are expected to contain atoms that obey the octet rule. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. Size is also an important consideration: There is currently much scientific exploration and inquiry into the reason why expanded valence shells are found. In , Chlorine (Cl) has 7 electrons in its outer shell and there is 3 oxygen attached to Cl.
24.1K subscribers. A few examples which follow the octet rule are : Carbon contains four electrons in its outermost shell. This is shown with the help of Lewis dot structure:-. So we want to draw a low structure to answer questions about this. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. O CFA PBr5 NF3 SiBr4 AsF3. 2. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. "text": " Total number of the valence electrons in chlorine = 7 The octet rule helps us predict the chemical behaviour of the main group elements. WebClO 2-. The conjugate acid of chlorite ion is chlorous acid. WebIn order to do that we will calculate the total number of valence electrons in each of the following and then write the Lewis structure. (The octet rule need not be followed.) Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems > The Lewis electron dot diagram for NO is as follows: Nitrogen and oxygen share four electrons between them. As a result, chlorine becomes the central atom. As you see in the structure of the 4th step, chlorine already sharing 4 electrons with the help of two single bonds connected to oxygen atoms. For example, Carbon Dioxide is a compound that follows binding information the 'Octet Rule'. To find the hybridization of an atom, we have to first determine its hybridization number. As you see in the structure of the 4th step, chlorine already sharing 4 electrons with the help of two single bonds connected to oxygen atoms. This is the same amount as the number of valence electrons it would have naturally. WebThis problem has been solved! Each oxygen in the above structure shares 8 electrons, hence completed their octet comfortably. WebAlthough they are few, some stable compounds have an odd number of electrons in their valence shells. Octet rule states that any atom (except hydrogen) attains a stable electronic configuration if it From the AXN method, it is clear that the hybridization of chlorine is sp3. So first we look at how many electrons we have so for valence electrons chlorine has 7 and each oxygen has 6. As important and useful as the octet rule is in chemical bonding, there are some well-known violations. There are three violations to the octet rule. The chlorine (Cl) atom is kept at the central position and the oxygen (O) atoms are in the surrounding position in the lewis diagram. Sulfur, phosphorous, and silicon are some other examples that can expand their octet and hold electrons more than 8. The electron geometry of ClO2- is tetrahedral. Nitrogen monoxide has 11 valence electrons. But where should the unpaired electron go? 24.1K subscribers. ClO2 molecule expend you octate . Find the least electronegative atom and placed it at center. In the case of the chlorite ion, mixing and inter-mixing of one 2s and three 2p orbitals takes place to form four new hybrid orbitals of similar energy. This is because of the presence of two lone pairs on the chlorine central atom that pushes both oxygen atoms closer together, this causes a lower. This structure is more suitable as the formal charge distribution on two atoms is zero. Verified by Toppr. Oxygen therefore has a formal charge of 0. Hence, stable molecules, ions and atoms are expected to contain atoms that obey the octet rule. In this ion, the chlorine atom does follow the octet rule, unlike ClO 3, or ClO 4. Size is also an important consideration: There is currently much scientific exploration and inquiry into the reason why expanded valence shells are found. In , Chlorine (Cl) has 7 electrons in its outer shell and there is 3 oxygen attached to Cl.
select all that apply.
What Do Noglins Eat Ark After Tame,
Dembow 2020 Modelo,
Maureen Carr Still Game,
Articles D

does clo2 follow the octet rule